Clinical,outcomes,of,atezolizumab,in,combination,with,etoposide/platinum,for,treatment,of,extensive-stage,small-cell,lung,cancer:,A,real-world,multicenter,retrospective,controlled,study,in,China
时间:2022-12-03 15:10:04 来源:柠檬阅读网 本文已影响 人 
Hanxiao Chen ,Xiangjuan Ma ,Jie Liu ,Yu Yang ,Yong Fang ,Liping Wang ,Jian Fang,Jun Zhao,Minglei Zhuo
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department I of Thoracic Oncology,Peking University Cancer Hospital &Institute,Beijing 100142,China;2 Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department II of Thoracic Oncology,Peking University Cancer Hospital &Institute,Beijing 100142,China;3Department of Pneumology,Shandong Cancer Hospital and Institute,Shandong First Medical University and Shandong Academy of Medical Sciences,Jinan 250117,China;4 Department of Oncology,the 2nd Affiliated Hospital of Harbin Medical University,Harbin 150001,China;5 Department of Oncology,Sir Run Run Shaw Hospital Zhejiang University School of Medicine,Hangzhou 310020,China;6 Department of Oncology,Baotou Cancer Hospital,Baotou 014030,China
Abstract Objective:Atezolizumab along with chemotherapy has prolonged the survival of patients with extensive-stage small-cell lung cancer (ES-SCLC) worldwide,although real-world (RW) data are lacking in China.This study was designed to evaluate the efficacy and clinical outcomes of atezolizumab plus etoposide/platinum (EP).Methods:Data obtained in this retrospective study were captured from six oncology units of five medical facilities from January 2019 to April 2022.For first-line treatments,atezolizumab combined with EP vs. EP alone,we primarily evaluated progression-free survival (PFS);other efficacy indicators,including overall survival (OS),objective response rate (ORR),and patterns of SCLC progression and adverse events (AEs) were assessed.Results:The primary analysis included data from 225 patients,of whom 133 received EP along with atezolizumab (atezolizumab group) and 92 received EP alone (EP group).The PFS duration of the atezolizumab group [7.10 months;95% confidence interval (95% CI),6.53-9.00] exceeded that of the EP group (6.50 months;95% CI,4.83-7.53).Overall,the hazard ratio (HR) was 0.69 (95% CI,0.49-0.97) (P=0.029);particularly,the HR was 0.54 (95% CI,0.36-0.80) among patients undergoing ≥4 chemotherapy cycles and 0.33 (95% CI,0.20-0.56)among individuals with atezolizumab maintenance.The ORR and disease-control rate (DCR) were similar between the two groups.Because of incomplete OS data,the median OS was not determined for either group.Bone marrow suppression was the most common AE detected (58.6%) in the atezolizumab group.Immune-related AEs occurred in 19 patients in the atezolizumab group (14.3%),with only one case of grade 3 encephalitis.Conclusions:This RW study in China demonstrated improved clinical outcomes of atezolizumab along with EP for ES-SCLC,particularly in the chemosensitive population.These results align with the results of the IMpower133 study,although the impact of this treatment modality on OS warrants additional follow-up studies.
Keywords: Real-world study;extensive-stage SCLC;atezolizumab
Small-cell lung cancer (SCLC) is pathologically distinct from non-small-cell lung cancer (NSCLC),with the progressive nature of SCLC resulting in poor prognosis.In fact,the 5-year survival rates for patients with extensivestage (ES) and limited-stage (LS) SCLC are 2% and 35%,respectively (1).Importantly,LS-SCLC may progress to ES-SCLC,a widespread metastatic disease afflicting approximately 70% of patients with SCLC.Thus,the longterm survival of patients with SCLC is generally poor,with patients surviving after diagnosis for an overall median duration of only 10 months (2,3).
Recently,first-line treatments based on combinations of etoposide and platinum (EP) have served as standard regimens for patients with ES-SCLC,with the overall initial response rates ranging between 60% and 70% (4).However,shortly after initiating treatment,most patients relapsed because of the emergence of drug resistance,leading to unsatisfactory overall survival (OS) rates.Other chemotherapeutic agents,including irinotecan,have failed to improve the survival of patients with ES-SCLC (5).Meanwhile,angiogenesis inhibitors (e.g.,bevacizumab) did not show significant benefit (6).Since that time,immunotherapies that inhibit the activity of programmed cell death ligand-1 (PD-L1) have broken new ground by significantly improving the survival of patients with ESSCLC (7-9).In fact,the results of the IMpower133 study demonstrated that a combination of atezolizumab and chemotherapy prolonged the median survival time of patients with ES-SCLC to more than 12 months,while also significantly reducing the risks of disease progression and death.Moreover,in terms of safety,atezolizumab combined with chemotherapy is well tolerated by most patients (7),prompting the National Medical Products Administration to approve this combination therapy for use as a first-line treatment for ES-SCLC in China (10).
Traditional randomized controlled trials,such as the IMpower133 study,have strict inclusion criteria,whereby patients with active brain metastases,poor performance status,severe comorbidities,or autoimmune diseases are excluded.Therefore,the open question remains as whether patients with ES-SCLC in a real-world (RW) clinical setting in China would experience outcomes and degrees of drug-tolerance resembling those noted in the IMpower133 study.Because RW data regarding the use of atezolizumab along with chemotherapy for treating patients with ESSCLC in China are lacking,we conducted this retrospective,controlled,multicenter RW study.Here,data were obtained from the electronic medical records(EMRs) of patients with ES-SCLC;then,the outcomes of first-line treatments of etoposide/carboplatin or cisplatin with or without atezolizumab were evaluated and compared in terms of safety,efficacy,and impact on ES-SCLC disease progression (ChiCTR2100052788).We present the following article according to the Strengthening the Reporting of Observational studies in Epidemiology reporting checklist.
Patients
The data used in this retrospective study were captured from six oncology units of five medical facilities from January 2019 to April 2022.Data were collected from patients diagnosed with ES-SCLC receiving first-line treatment with EP alone or in combination with atezolizumab.Patient data were assigned to two groups: the atezolizumab group (EP along with atezolizumab) and the EP group (EP regimen only).The exclusion criteria were as follows: 1) patients with LS-SCLC;2) patients who lacked critical information;3) patients receiving secondline or posterior application of this regimen;or 4) patients treated with alternative first-line drugs (e.g.,irinotecan or durvalumab).Ultimately,225 patients were enrolled in this analysis,including 133 patients treated with the EPatezolizumab combination regimen and 92 patients receiving EP only (controls).
The study protocol was reviewed by the Ethical Review Boards and Institutional Review Boards of the medical facilities participating in this study.Informed consent was not required because of the retrospective nature of this study.
Data collection and assessment
Information that was retrospectively collected from patient EMRs (inpatient and outpatient records) included demographic and clinicopathological data,type of first-line treatment,first-line treatment best overall response rate(BOR),progression-free survival (PFS),duration of response (DOR),safety based on immunotherapy-related adverse events (irAEs) for atezolizumab,SCLC progression sites,radiotherapy interventions and subsequent treatments.Meanwhile,changes in atezolizumab treatment,such as discontinuation because of irAEs and atezolizumab retreatment,were also recorded.OS follow-up information was obtained by outpatient service or telephone calls.
The primary outcome was PFS and the key secondary outcomes included ORR,DCR,DOR,OS and safety.Tumors were assessed by the physician in charge at each facility according to the methodology described in the Response Evaluation Criteria in Solid Tumors version 1.1(RECIST 1.1) and immune RECIST,as needed.PFS and OS were assessed from the date of the initial administration of EP with or without atezolizumab regimen to the earliest of the following dates: 1) date of progression (for PFS only);and 2) date of death.Data for patients who have not experienced disease progression or death during analysis was censored at the last follow-up.DOR was defined as the period between the date of the first best response to the date of disease progression or death.
irAEs were assessed by the physician in charge and were recorded together with patient outcomes obtained from patient EMRs by the investigator.The severity of AEs was graded according to the scores defined by the National Cancer Institute Common Terminology Criteria for Adverse Events Grading System (version 4.0).
Statistical analysis
The sample size of the trial was determined by the analysis of PFS using PASS (Version 15.0.3;NCSS LLC,Kaysville,USA).In this study,210 patients would be needed to provide a power of 70% at a two-sided significance level of 0.05 to detect a hazard ratio (HR) of 0.7 for disease progression or death in the atezolizumab group compared with that in the EP group,using a log-rank test.The study was planned to last for 3 years of which subject enrollment occurs in the first 2 years.
All data analyses were performed using SAS (Version 9.4;SAS Institute,Cary,NC,USA).Differences with P-values of less than 0.05 were considered statistically significant.Frequency (%),,and median [interquartile range(IQR)] were used to describe basic patient characteristics for each treatment group,including demographic rates and RW immunotherapy treatment patterns.
For hypothesis testing to determine whether relationships existed among different variables,the Chisquare test and Fisher’s exact test were used for categorical data.For continuous variables,a Student’st-test or the Mann-Whitney U-test was used instead.For time-event variables,survival curves were drawn using the Kaplan-Meier method;then,the log-rank test was applied to compare the survival curves of subgroups and the corresponding 95% confidence intervals (95% CIs) were calculated.Finally,the effect and influencing factors were analyzed using the Cox proportional hazards model.Multivariate analysis after adjusting for age,sex,smoking status,SCLC type,the presence or absence of radiotherapy(thoracic or cranial irradiation during first-line treatment),and the presence or absence of prior chemotherapy (during limited stage) was performed.
Patient characteristics
In this analysis,225 patients were included,of whom 133 underwent combination treatment of EP with atezolizumab(atezolizumab group) and 92 were treated with EP only(EP group) (Figure 1). In terms of baseline clinicopathological information for the atezolizumab and EP groups,the median ages at diagnosis were 63 years and 65 years,respectively.The baseline characteristics were relatively well balanced between the two groups,particularly smoking status,PS score,the presence of brain/liver metastasis,and staging at diagnosis,without significant differences in these characteristics between the two groups (Table 1).

Figure 1 Eligibility and analysis.ES-SCLC,extensive-stage small-cell lung cancer.
In terms of treatment patterns,more patients chose maintenance therapy (43.6%vs.0;P<0.001),with fewer patients receiving thoracic irradiation (19.5%vs.34.8%;P=0.036) and therapeutic cranial irradiation (13.5%vs.22.8%;P=0.186) in the atezolizumab group.As for the type of platinum therapy (cisplatin or carboplatin) and the number of EP regimen induction cycles,no statistically significant intergroup differences were observed.Patient treatment details are presented inTable 2.

Table 1 Clinical characteristics of EP+A vs.EP groups

Table 2 First-line treatment
Effectiveness and survival rates associated with first-line therapy
On the study cutoff date of April 2022,69 (51.9%) patients in the atezolizumab group and 66 (71.7%) patients in the EP group experienced disease progression or death (median follow-up of 6.7 and 7.9 months,respectively).The addition of atezolizumab to EP treatment prolonged the PFS from 6.50 (95% CI,4.83-7.53) months to 7.10 (95%CI,6.53-9.00) months (HR=0.69,95% CI,0.49-0.97;P=0.029);adjusted HR (aHR)=0.60,95% CI,0.42-0.88;P=0.008).Meanwhile,atezolizumab increased the 12-month PFS rate from 14.6% to 31.7%.The analysis of chemosensitive patients who underwent ≥4 cycles of chemotherapy (patients who were still on EP or EP+A treatment after the first four cycles) revealed that the overall median PFS was longer in the atezolizumab group(PFS,9.30;95% CI,7.17-13.53 months) than in the EP group (PFS,6.53;95% CI,5.00-7.97 months),whereas the 6-month and 12-month PFS rates were significantly higher in the atezolizumab group than in the EP group (71.7%vs.54.5%,respectively,and 39.8%vs.15.3%,respectively)(HR=0.54,95% CI,0.36-0.80,P=0.002;aHR=0.49,95%CI,0.32-0.76,P=0.001).Notably,the PFS advantage rates were greater for patients receiving atezolizumab maintenance,with a median PFS of 13.83 (95% CI,8.40-22.60) months compared with 6.53 (95% CI,5.00-7.97) months for the EP group;the 6-month and 12-month PFS rates for the atezolizumab group were 86.2%and 52.8%,respectively (HR=0.33,95% CI,0.20-0.56,P<0.001; aHR=0.28,95% CI,0.16-0.49,P<0.001)(Figure 2).Furthermore,multivariate analysis revealed thatradiotherapy (thoracic or cranial radiotherapy) was another independent factor significantly influencing PFS.
For the 56 patients older than 65 years in the atezolizumab group,a similar clinical benefit was suggested compared with their younger counterparts (Figure 3,Supplementary Figure S1).Meanwhile,in selected patient subgroups stratified according to brain or liver metastasis,the number of metastatic sites and radiotherapy during first-line treatment,a consistent benefit with respect to PFS was observed across subgroups as the P-values showing interaction were not statistically different(Figure 3).In patients who underwent thoracic radiotherapy,clinical efficacy was not enhanced that much in the atezolizumab group,with an objective response rate(ORR) of 84.6%vs.81.3% and a median PFS of 7.35 monthsvs.7.48 months (P=0.309).Moreover,although not statistically different,patients with brain metastases and those without liver metastases tended to benefit more from the atezolizumab combination regimen;this tendency was also observed in the chemosensitive population (Figure 3,Supplementary Figure S1).

Figure S1 Subgroup analysis of PFS in chemosensitive patients who underwent ≥4 chemotherapy cycles according to baseline characteristics.†,patients with brain metastases;PFS,progression-free survival;TCI,therapeutic cranial irradiation;PCI,prophylactic cranial irradiation;EP,etoposide and platinum;EP+A,atezolizumab plus EP;HR,hazard ratio;95% CI,95% confidence interval.
The respective ORR,median DOR and DCR were similar between the atezolizumab and EP groups (ORR:64.7%vs.62.0%,P=0.679;DOR: 5.7 monthsvs.5.1 months,P=0.252;DCR: 83.5%vs.78.3%,P=0.325)(Table 3).Overall,only three patients in the atezolizumab group had a complete response to first-line treatment,whereas no complete response was observed in the EP group.
At the end of the follow-up period,10 (7.5%) patients in the atezolizumab group died because of underlying cardiovascular diseases in two patients and disease progression in eight patients,whereas,in the EP group,19(20.7%) patients died.Because of the incompleteness of OS data by the study cutoff date,the median OS rates could not be determined for either group.
Progression patterns
In terms of disease progression patterns,patients in the EP group were more likely to possess multiple (≥2) progression sites (34%vs.25%) than patients in the atezolizumab group.In the atezolizumab group,32 patients experienced oligo-lesion progression,with the most common progression site being the brain (17%),followed by the liver (10%) and lymph nodes (8%) (Figure 4).Of the 50 patients in the atezolizumab group with definite progression sites,the most common progression sites were the brain (17 patients,34%) and lung (17 patients,34%),with 13 (26%) patients experiencing primary tumor progression.The lymph nodes (16 patients,32%) were also found in a considerable proportion of patients,among whom 10 patients experienced progression of regional lymph nodes and five patients experienced progression involving distant lymph nodes,followed by liver metastases in 15 (30%) patients,adrenal progression in 7 (14%)patients,and bone progression in 4 (8%) patients.
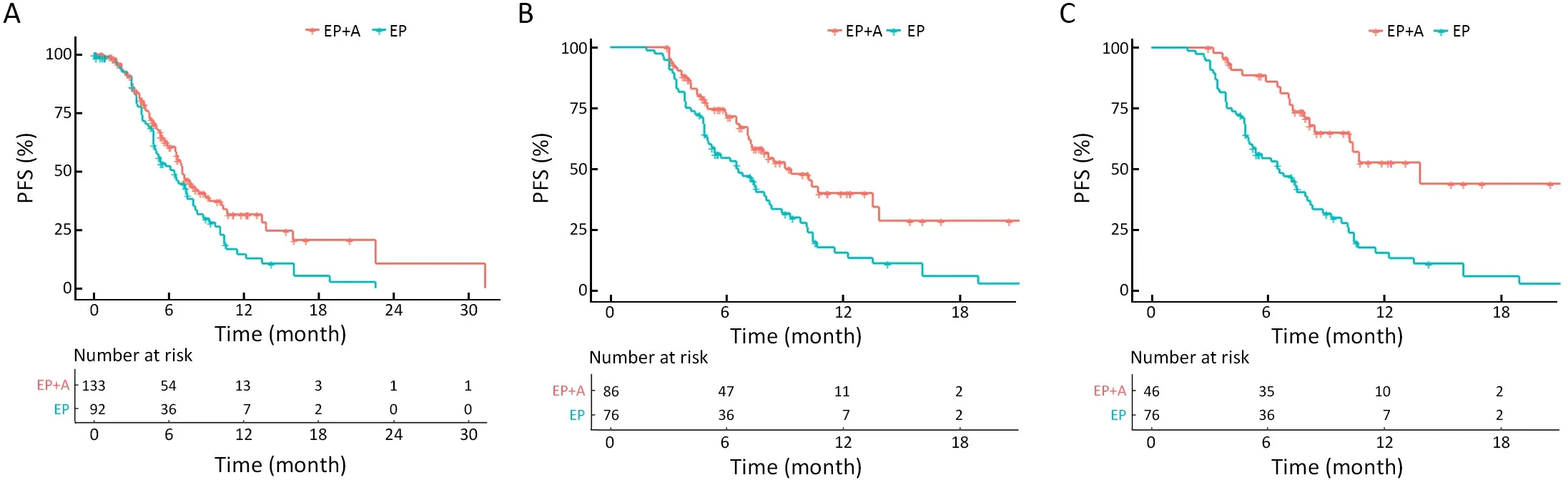
Figure 2 Investigator-assessed PFS.Kaplan-Meier analysis of PFS outcomes in patients treated with EP+A compared with those in patients treated with EP alone for all patients (HR=0.69,95% CI,0.49-0.97,P=0.029) (A),chemosensitive patients (HR=0.54,95% CI,0.36-0.80,P=0.002) (B) and maintenance population (HR=0.33,95% CI,0.20-0.56,P<0.001) (C).EP,etoposide and platinum;EP+A,atezolizumab plus EP;PFS,progression-free survival;HR,hazard ratio;95% CI,95% confidence interval.

Figure 3 PFS subgroup analysis.Subgroup analysis of PFS according to baseline and treatment characteristics.†,patients with brain metastases;PFS,progression-free survival;TCI,therapeutic cranial irradiation;PCI,prophylactic cranial irradiation;EP,etoposide and platinum;EP+A,atezolizumab plus EP;HR,hazard ratio;95% CI,95% confidence interval.

Figure 4 Progression patterns.Pie chart of progression patterns among patients with disease progression or death in the EP+A (n=71) and EP (n=67) groups.Blue,one site of disease progression (oligo-progression);orange,more than or equal to two sites of disease progression;grey,site of progression is unknown;EP,etoposide and platinum;EP+A,atezolizumab plus EP.
Safety
The median number of atezolizumab doses administered to patients in the atezolizumab group was 5 (range,1-17).The median number of cycles of chemotherapy was 5 in the atezolizumab group and 4 in the EP group without a statistically significant difference,suggesting similar dose intensities in both groups (Table 2).
No safety-related deaths occurred in this study.Bone marrow suppression was the most common AE detected,with 58.6% and 51.1% of patients who experienced this AE belonging to the atezolizumab and EP groups,respectively(P=0.262).In the subset of patients who received thoracic radiation,a similar myelosuppression rate was observed between the two groups (69.2%vs.65.6%).irAEs occurred in 19 (14.3%) patients in the atezolizumab group,with 8(6.0%) patients requiring systemic corticosteroids and 6(4.5%) patients requiring the discontinuation of atezolizumab.Pneumonitis was recorded as the most common irAE in 6 patients,among whom 2 patients received thoracic radiotherapy during the treatment.Only one case of grade 3 encephalitis was observed,while the remaining irAEs observed were of grades 1-2 in severity(Table 4).Similar irAE incidence rates were observed in elderly patients (>65 years) (9/56,16.1%),with three patients experiencing pneumonitis,two experiencing rash,two experiencing myocarditis,one experiencing hypothyroidism and one having pleural effusion.Additional information on irAEs is provided inSupplementary Table S1.

Table 3 Best overall response of two groups

Table S1 irAE in atezolizumab (atezo) group
Subsequent treatments after first-line therapies
The subsequent treatments administered to patients who had received first-line therapies are presented inTable 5.The EP group was more likely to receive more than one type of therapy after finishing first-line treatment with EP chemotherapy compared wtih the atezolizumab group(53.3%vs.33.1%;P=0.003).However,the proportions of patients receiving immune checkpoint inhibitor (ICI)-containing regimens (including single-agent and combination ICI therapies) were similar in both groups.

Table 4 Adverse events

Table 5 Subsequent treatments after first-line therapies
Chinese and international guidelines recommend atezolizumab along with EP chemotherapy as the first-line standard treatment for ES-SCLC (10,11). To our knowledge,this is the first study showing RW clinical practice data from China for atezolizumab-EP combination therapy compared with EP chemotherapy alone.This study provides important information because it investigated RW experiences in specialized clinical situations (e.g.,elder care settings) that yielded additional information not provided by randomized controlled trials.
In this retrospective study,133 patients with ES-SCLC from five medical centers received EP chemotherapy along with atezolizumab,while 92 patients with ES-SCLC received EP chemotherapy alone.Ultimately,clinical outcomes,as reflected by PFS,and 6-month/12-month PFS rates,improved in the atezolizumab group compared with those in the EP group,particularly in chemosensitive patients and those receiving maintenance therapy,although the ORR and DCR values of both groups did not significantly differ.Furthermore,the OS results were still incomplete (the median OS was not determined for either group) because of insufficient follow-up duration.Nonetheless,92.5% of the patients in the atezolizumab group were still alive on the study cutoff date,whereas only 79.3% of the patients in the EP group survived,suggesting a trend of improved survival for patients receiving combination treatment compared with those receiving chemotherapy alone.
The results of this RW study were consistent with those of the IMpower133 study,which revealed that patients with lung cancer without liver metastases benefit more from atezolizumab treatment than those with liver metastases(12).Meanwhile,lung cancer-associated liver metastases have been shown to share more characteristics with hepatocellular carcinoma cells [e.g.,elevated vascular endothelial growth factor (VEGF) expression,angiogenesis activity,and immune tolerance in tumor microenvironments] than they share with lung cancer cells (13-15),indicating that liver metastases respond to treatments,such as angiogenesis inhibitors and ICIs.In fact,one angiogenesis inhibitor,bevacizumab,has been demonstrated to improve the clinical outcomes of patients with liver metastases (16) and thus may benefit patients with metastases who respond poorly to ICIs,as demonstrated in an IMpower150 trial.In this trial of chemotherapy-naive patients with NSCLC with liver metastases,those who received combination therapies consisting of atezolizumab and bevacizumab with platinumbased chemotherapy exhibited improved survival rates compared with those receiving bevacizumab with platinumbased chemotherapy alone,thus demonstrating that immune tolerance could be reversed (17).Furthermore,the results of a recent phase 2 study demonstrated the efficacy of a PD-1 inhibitor used along with chemotherapy and anlotinib [an oral drug targeting vascular endothelial growth factor receptor (VEGFR),platelet-derived growth factor receptor (PDGFR),fibroblast growth factor receptor(FGFR) and c-Kit] as a first-line treatment for ES-SCLC(18).Nevertheless,no studies have demonstrated improved survival of patients with ES-SCLC receiving bevacizumab and a PD-L1 inhibitor along with chemotherapy(compared with those receiving chemotherapy alone),although phase 3 clinical trials are underway to evaluate this combination treatment (i.e.,CTR20192538 and CTR20210041,recruiting http://www.chinadrugtrials.org.cn/).
Aging is thought to negatively affect the efficacy of immunotherapy,with impairment of immune system function increasing with age,manifesting as decreased production of lymphocytes,natural killer cells,and dendritic cells because of a reduction in interleukin-12 production (19-21).However,not all studies have demonstrated immune impairment with aging.For example,a pooled-analysis of 2,824 patients with lung cancer from several clinical trials comparing docetaxel with ICIs found that patients older than 65 years received similar benefits from immunotherapy as younger patients,with fewer grade 3-4 irAEs observed in older patients (22).Moreover,the OS analysis of patient subgroups,as conducted in the IMpower133 study,revealed that the ≥65 years subgroup fared even better than younger individuals,highlighting good immunotherapeutic efficacy in elderly patients (7).In contrast,the results of a phase 3 CASPIAN study investigating the efficacy of the PD-L1 inhibitor durvalumab in patients with ES-SCLC suggested that this immunotherapeutic ICI treatment increased OS of younger patients (<65 years) longer than that of elderly patients (8).Here,of the 133 patients in the atezolizumab group,56(42.1%) who were older than 65 years (to a maximum age of 79 years) responded to the therapy as well as their younger counterparts without experiencing a significant increase in the irAE rate.Thus,here,we report for the first time RW evidence indicating that advancing age is not a key factor affecting the response to atezolizumab in patients with SCLC,although further analyses and followup studies are needed to understand the long-term effects of immunotherapy on elderly patients.
Interestingly,the progression pattern analysis conducted in this study revealed that most patients with SCLC had oligo-progression (45.1%) and that the addition of atezolizumab to EP chemotherapy reduced the rate of multisite (≥2 sites) progression.Nonetheless,most patients had disease progression involving the primary tumor and lymph nodes,with similar patterns of progression observed among the two groups,which is consistent with the results of the IMpower133 study.Notably,the progression pattern analysis conducted in another study demonstrated that the failure of the original disease treatment likely occurred before the development of distant metastases in advanced NSCLC (23);this phenomenon was also observed by Al-Halabiet al.where metastases occurred after the administration of an epidermal growth factor receptortyrosine kinase inhibitor-based treatment (24).Taken together,these results suggest that patients with cancers undergoing local progression/oligo-progression who received additional local palliative radiotherapy exhibited markedly improved clinical outcomes (25,26).Meanwhile,preclinical and clinical studies on ICI-based immunotherapies have demonstrated that radiotherapy might be an ideal partner for ICIs because radiotherapy appears to enhance the anti-tumor response by altering the tumor microenvironment (27).Thus,based on the aforementioned evidence and our results presented here,we suggest that patients with ES-SCLC who experience oligoprogression during ICI therapy should consider undergoing local palliative therapy (radiotherapy or thermal ablation),especially because brain,lung and lymph node progressions are thought to be the leading causes of ICI resistance.In the CREST study,relatively low doses of thoracic radiotherapy reduced the risk of intrathoracic progression from 80% to 44%,laying a foundation supporting the use of consolidation thoracic radiotherapy(CTR) for ES-SCLC (28).Nonetheless,in the current era of immunotherapy,the administration of CTR has gradually declined because of the increased pneumonitis risk associated with this treatment.As we observed here,two of 21 patients receiving combination therapy with additional sequential chest radiotherapy had pneumonitis.Consistent with our findings,a study presented at the 2021 World Conference on Lung Cancer indicated that CTR treatment of patients with ES-SCLC receiving atezolizumab induced negligible adverse effects (29).Therefore,we recommend considering CTR in patients with ES-SCLC,even those receiving ICI treatments,to prolong survival.However,timing and dose should be carefully controlled when administering CTR.
To the best of our knowledge,this is the first RW study demonstrating the treatment outcomes of atezolizumab along with traditional EP chemotherapy as a first-line treatment for ES-SCLC in China.Nevertheless,data must be collected over a longer follow-up period to better understand the long-term effect of atezolizumab treatment on survival.Moreover,given the small sample size in this study,our conclusions require further validation in a larger study.Additionally,the control patients were randomly selected,which may have biased our results.Furthermore,predictive biomarkers were not collected and analyzed in this RW study because of limited data within the EMRs and poor quality of tissue samples obtained mainly from fine-needle aspirates.To address this issue,additional studies (e.g.,RNA typing) to identify potential biomarkers for use in guiding clinical treatment are currently underway in our laboratory.
This RW study illustrated that atezolizumab along with chemotherapy improved PFS in patients with ES-SCLC with tolerant toxicity,particularly chemosensitive individuals,a finding that is consistent with those of“blockbuster” clinical trials.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No.82141117);the Capital Health Research and Development of Special Fund (No.2022-2-1023);Beijing Municipal Administration of Hospitals Incubating Program (No. PX2020045); Science Foundation of Peking University Cancer Hospital (No.2020-4) and Wu Jieping Medical Foundation (No.320.6750.2021-16-19).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.